【News】International medical journal publishes article showing simultaneous effects of improving gait function and reducing muscle breakdown with Medical HAL for Spinal and Bulbar Muscular Atrophy (SBMA)
The article, “The Combined Efficacy of a Two-Year Period of Cybernic Treatment With a Wearable Cyborg Hybrid-Assistive Limb and Leuprorelin Therapy in a Patient With Spinal and Bulbar Muscular Atrophy: A Case Report” was published in the international journal Frontiers in Neurology in June 2022. The corresponding author of this paper was Dr. Takashi Nakajima (Director, National Hospital Organization Niigata Hospital), and the paper reported the effects of Cybernics Treatment with the Medical HAL Lower Limb Type on patients with Spinal and Bulbar Muscular Atrophy*1, a slowly progressive neuromuscular intractable disease. This paper reports that the addition of Cybernics Treatment with HAL to treatment with Leuplin*2 improves gait function in slowly progressive patients, which had been considered difficult with conventional treatment methods, while simultaneously decreasing (improving) serum creatine kinase (CK) levels, which reflect neuromuscular damage, and was published as a groundbreaking achievement.
【URL link to the article】
https://www.frontiersin.org/articles/10.3389/fneur.2022.905613/full
【Summary of the article】
The article introduces the case of a 39-year-old man with SBMA who went through anti-androgen therapy with leuprorelin acetate*2, followed by Cybernics Treatment a year later.
In the presented case, Cybernics Treatment was performed for 20 to 30 minutes per session per day for nine sessions (1 course) over two weeks. After that, treatment with Leuplin was combined with Cybernics Treatment with Medical HAL every two months for two years (13 courses total).
According to the 2-minute walk test assessment, walking ability improved by 20.3% in the first course and reached its peak (59.0% improvement) 10 months after the start of combination therapy, and walking function was maintained throughout the treatment period.
It should be noted that SBMA is usually characterized by high serum creatine kinase (CK) levels, reflecting neuromuscular involvement, which is further elevated by exercise therapy. In this case, a significant decrease in CK levels with treatment was demonstrated, which was previously thought impossible.
This paper suggests that the combination of Leuplin (which can inhibit the progression of dysphagia but has not been proven to improve gait function) with Cybernics Treatment using Medical HAL may improve gait function without damaging the motor unit (consisting of motor neurons and the muscle fibers they control) and may inhibit disease progression in the long term.
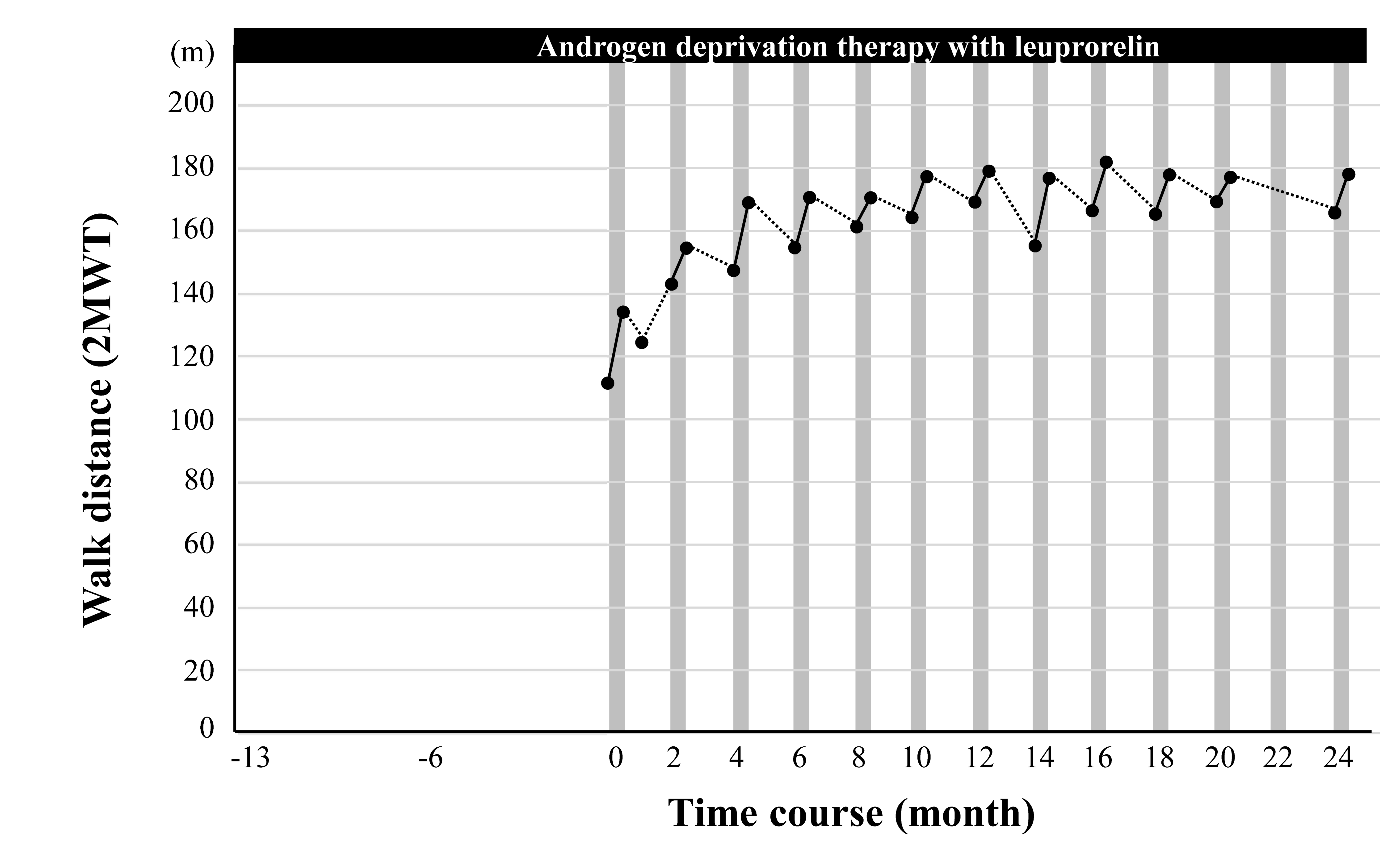
Effects of Cybernics Therapy with Medical HAL on Gait Function
Effects of Cybernics Therapy with Medical HAL on Gait Function
– Black dots indicate the measured distance walked in 2 minutes (2-minute walk test, 2MWT)
– The gray vertical bar indicates one course of Cybernics Treatment with HAL
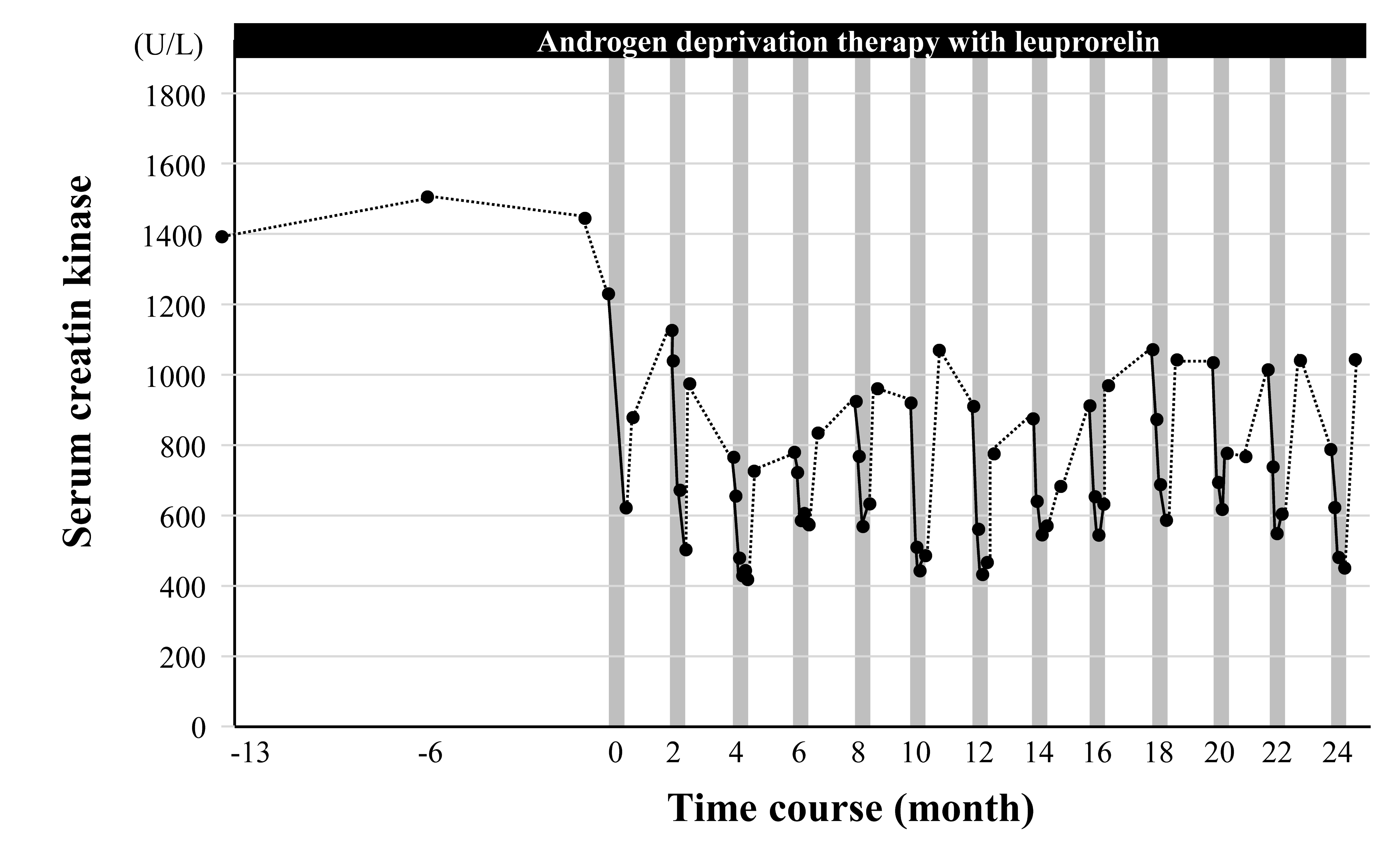
Effect of Cybernics Treatment with Medical HAL on creatine kinase
– Black dots indicate serum creatine kinase (CK) levels
– Gray vertical bars indicate one course of Cybernics Treatment with HAL
<Comment from Dr. Takashi Nakajima, Director of National Hospital Organization Niigata Hospital>
Based on observations of cases conducted at the National Hospital Organization Niigata Hospital, this study was submitted to Frontiers in Neurology. This international medical journal publishes peer-reviewed papers and records rigorous discussions and was accepted for publication as a case report after review. It is also a valuable paper that describes the detailed treatment mechanism of HAL.
This case report highlights the latest approved treatment for Spinal Bulbar Muscular Atrophy (SBMA), an intractable neuromuscular disease. The “quality of life” and “prognosis of life” in this disease depend on the degree of dysphagia and gait dysfunction, and the approved treatment for the etiology in Japan, leuprorelin acetate is effective for dysphagia but does not improve gait function.
Exercise therapy has traditionally been a concern for neuromuscular diseases because it can cause motor overexcitement and aggravate the condition. The clinical trial (investigator-initiated clinical trial) proved and reported that the innovative exercise treatment (Cybernics Treatment) using the Hybrid Assistive Limb (HAL) is safe and effective for eight neuromuscular diseases, including SBMA.
(https://pubmed.ncbi.nlm.nih.gov/34233722/)
This case report demonstrates that gait function can be improved and maintained long-term when HAL is used in combination with leuprorelin acetate treatment of SBMA. Furthermore, the serum CK level, a biomarker of muscle damage, was markedly reduced, breaking through the existing problems in exercise therapy and being a revolutionary treatment for the disease itself.
Therefore, it can be said that this combined therapy using HAL is not only a breakthrough in SBMA treatment itself but also suggests a solution for other neuromuscular diseases, such as spinal muscular atrophy and duchenne muscular dystrophy, in which motor function is not sufficiently improved by the causative treatment alone, as a combined treatment model.
Although the results of this study are a case report, the paper was highly regarded for its impact on treatment strategies for other neuromuscular diseases.
*1 Spinal and Bulbar Muscular Atrophy (SBMA for its acronym) SBMA is an inherited lower motor neuron disease that usually affects adult males. The main symptoms are muscle weakness, atrophy of the extremities, ball paralysis, complicated by gynecomastia and other mild androgen deficiency disorders, glucose intolerance, and dyslipidemia. Muscle weakness usually develops between the ages of 30 and 60, and the course of the disease is slowly progressive. The international name for this disease is Spinal and Bulbar Muscular Atrophy (SBMA), but it is also called Kennedy disease.
Sources: Japanese Intractable Disease Information Center (available in Japanese only)
https://www.nanbyou.or.jp/entry/234
*2 Leuplin SR (Takeda Pharmaceutical Company Limited, additional indication approved in August 2017)
Indication: suppression of the progression of spinal and bulbar muscular atrophy
Dosage and administration: The usual adult dosage is 11.25 mg of leuprorelin acetate administered subcutaneously once every 12 weeks.
Indications: Inhibition of the progression of spinal and bulbar muscular atrophy.
Dosage and Administration: The usual adult dosage is 11.25 mg of leuprorelin acetate administered subcutaneously once every 12 weeks.
Source: Takeda Pharmaceutical Company Limited
https://www.takeda.com/jp/newsroom/newsreleases/2017/20170828_7818/